Staff & Recruitment
NHSGGC Get Ready for Medicine Programme
What is the “Get Ready for Medicine” Programme?
The Get Ready for Medicine programme, developed in collaboration with the University of Glasgow Medical School, is a two-day programme that supports senior phase school pupils and adults applying for post-graduate or access programme entry schemes (eg SCOTGEM, SWAP) to apply to medical school. The intention is to deliver a meaningful programme of activity that will give participants more to speak about within their medical school application and at interview.
The programme is delivered by NHSGGC Consultants, Junior Doctors and University of Glasgow Medical Students in an NHSGGC Hospital.
Activities include:
Discussion workshops hosted by doctors, medical students and medical school staff:
- On the application process for medicine
- Life at medical school
- Life as a doctor
- Career pathways within medicine.
Hands on clinical skills workshops that include:
- Giving experience of blood taking
- Suturing (stitching)
- Patient observations
- Basic life support.
Can’t I just do a week’s work experience instead?
Medical work experience used to simply refer to “shadowing” doctors in the workplace – either in hospital or in a GP practice. It has now been recognised that far more important than “what you have done” is “what you have learned from it”.
Within NHS Greater Glasgow and Clyde all medical work experience now takes place as part of an organised programme.
Other Programmes
When can I apply for the Get Ready for Medicine Programme?
Get Ready for Medicine programmes will take place as follows:
August to September: Online evening lecture events & half day workshops for S6 pupils making applications in October
January to March: Online evening lecture events & half day work experience workshops for S5, gap year and access students and postgraduate applicants planning October applications
June to July: NHS GGC will support delivery of Medic Insight Glasgow Programme (please make sure you are following Medic Insight Glasgow social media platforms)
For up to date info please see our page : Get Ready For Programmes / Career Insights – NHSGGC
Get Ready for Medicine Work Experience Workshops.
This even open to anyone who is applying to medical school next October who hasn’t previously attended a GRfM workshop event.
We would also advise to make sure you have a look at the information below for the Reach Programme and Medic Insight Glasgow Programme. Also make sure are following Medic Insight Glasgow social media platforms ( ie Facebook) for the Medic Insight Glasgow Programme and some Guidance for aspiring doctors from current medical students. See more details below:
Please note that NHSGGC does not host “Shadowing” within a number of professions including Medicine. All requests for work experience, to source placements or support self found placements for Medicine will be re directed to the Get Ready for Medicine Programme.
Other Resources
We would also advise to make sure you have a look at the information below for the Reach Programme and Medic Insight Glasgow Programme. Also make sure are following Medic Insight Glasgow social media platforms (i.e. Facebook) for the Medic Insight Glasgow Programme and some Guidance for aspiring doctors from current medical students. See more details below:
You can visit the Becoming a Doctor webpage and YouCanBeADoctor to view content that may help you gain insight and support your application to study medicine.
Other Programmes
Reach Programme
Reach is a national project funded by the Scottish Funding Council (SFC) which aims to support eligible S4-S6 pupils in local state secondary schools. who are considering pursuing degrees or careers in law, medicine or veterinary medicine.
Pupils’ participation in the programme takes place over the three years of their senior phase (S4 to S6), and covers everything from introducing pupils to medicine in S4 to supporting their applications to University to study medicine in S6.
Scotland has five medical schools: Glasgow, Edinburgh, St Andrews, Dundee and Aberdeen and all five take part in Reach, specifically focused on widening access to medical degrees to students from Scotland’s poorest neighbourhoods.
The Reach programme will offer you encouragement, and impartial advice on medicine as a career, as well as give you guidance on school subject choices.
They will also help you with all aspects of the UCAS application process.
Medic Insight
Medic Insight is a programme that offers week-long or day events to fourth and fifth-year school students in Scotland who are interested in becoming doctors. The programme allows the opportunity for these students to sit in on consultations, go to theatre and provide access to a wide range of specialities and levels of clinicians in a hospital setting.
There are programmes in Glasgow, Edinburgh and Dundee. If you would like more information or wish to apply, please visit the Medic Insight Facebook pages:
Medic Insight Edinburgh.Facebook Page
Work experience requirements when applying to study medicine
When applying to medical school the important thing is not so much what you have done for work experience but what you have learned from it and how that has given you a better understanding into the career.
That means that someone who has never been into a hospital but has read lots, spoken to healthcare staff and maybe done some volunteering or worked a part-time job dealing with the public, and who can talk about these things widely, may perform much better at application and interview than someone who has spent many days shadowing doctors but who cannot describe what this taught them about being a doctor, working in the NHS and looking after patients.
Read what the Medical Schools Council advise on the type of work experience needed to support your application to study Medicine.
The Royal College of General Practitioners (RCGP) has launched a free online platform, Observe GP, designed to support aspiring medics in making informed career choices and in preparing their application for medical school.
The General Medical Council (GMC) also offer a number of online resources which will help you gain insight into role of a Doctor including a virtual reality Patient Journey in a GP Practice
Can I get work experience in a Hospital?
Rather than ad hoc work shadowing or work experience for medicine, NHS Greater Glasgow and Clyde delivers their Get Ready for Medicine Programme in collaboration with the University of Glasgow School of Medicine.
___________________________________________________________________________________________________
So you have decided to study Medicine…
_____________________________________________________________
Main workstreams
_________________________________________________________________________________________________
____________________________________________________________
Contact the Employability Team
_________________________________________________________________________________________________________
Further Information
Was this helpful?
While watching videos cannot fully replace real-world experience and conversations with health professionals, we have created some resources to guide your own reflections on what you are seeing. By working through these you will take away some important learning points about how the whole team interacts in a hospital environment and how both patients and staff may feel about certain scenarios.
How to use these resources
For each video we have set a number of questions in the documents attached. We would encourage you to come up with your own answers first, and then to look through our thoughts beneath. Remember – there is rarely a “right” or a “wrong” answer but the truth normally lies somewhere in the middle. It is important to have a reason for any answer you give however so that you can back it up if challenged on it at interview.
Remember also that our own “answers” here are far from complete and are simply designed to stimulate further thought and reading around the topics. For some of the videos we also suggest which supplementary “student interviews” are worth watching and are related to that particular scenario.
We hope you enjoy the videos and find these reflection materials useful and thought provoking.
Introduction to Virtual Ward Rounds
Chest Pain and Learning How to Perform Procedures
Leg Cellulitis and Taking a Patient Medical History
Wrist Fracture and Bedside Teaching
Drug Prescribing and Management of Errors
Acute Asthma and Simulation Training
Multi Disciplinary Team Work
Was this helpful?
It is almost impossible to describe “life as a doctor”. After finishing medical school the potential career pathways are so varied that no two doctors follow the exact same route.
There are obviously the roles which are well known and most commonly seen in the media – for example General Practice, Accident and Emergency, Surgery, Paediatrics, but there are also a huge number of other specialties which are equally important but less visible such as laboratory specialties (pathology, biochemistry, microbiology), radiology, and occupational health. Each role has its own necessary skills and so there really is a potential job for everyone within medicine.
Remember that no matter what specialty you pursue there will be further studying and exams and it often takes around 10 years after graduation from medical school before being a fully qualified specialist in your particular field.
Below are a number of videos from doctors in a range of specialties to give a flavour of the career after medical school.
Sources – You Can Be A Doctor, NHS Education for Scotland, NHS Lothian, Golden Jubilee National Hospital and NHSGGC/MOGWAI
Being a Junior Doctor in Scotland
Roberta: Trainee General Practitioner (GP)
Andrew: Consultant Anaesthetist Critical Care
Accordion item1
Thom: Paediatrics Clinical Fellow/Clinical Research
Hazel: Consultant in Older Peoples Medicine
Colin: Consultant Nephrologist (Kidney Specialist)
Kathleen: Trainee in Palliative (End of Life) Care
Adam: Consultant Anaesthetist Obstetrics
Nat and Thalia: Foundation Doctors in Acute Receiving
Dr Robot: Medicine and Technology
Was this helpful?
So you have decided to study medicine
Currently there are around 10 applicants for every available place at Medical School and achieving the grades required for entry is not enough. Your personal qualities are just as important as your academic ability and medical schools want applicants to show evidence of commitment, ability to work effectively under pressure, team-working skills, leadership and compassion.
The following resourses are designed to support you in your journey to Medical School.
Do I have the right grades in the right subjects?
The entry requirements for entry to each university can vary but excellent grades in science subjects such as chemistry and biology are essential.
Generally speaking you are aiming to achieve at least 5 Highers, usually at AAAAB or AAABB grades in S5 and SQA Advanced Highers at AB or BBB in S6, however consideration will be given to factors which may affect you achieving these grades.
Scottish Medical Schools are committed to ensuring that a person’s background or life circumstances are not a barrier to them studying medicine.
This means that Medical Schools will consider all circumstances which may prevent you meeting their standard entry requirements and make adjusted offers of entry accordingly.
This process is called contextualised admission and consideration is given to potential barriers such as disability, care experience*, carer responsibilities, refugee status and challenging financial or family circumstances. View more about the Adjusted Entry Criteria for the University of Glasgow.
- Care experienced applicants are people who live/have lived with foster parents/kinship carers or who live/have lived in a residential children’s setting/secure unit.
I’m not a school leaver and I don’t have these highers – can I still apply?
You can find out more about applying to study medicine and links to specific entry requirements for each university, including other accepted academic qualifications.
You may also apply to study medicine through the ScotGEMS Graduate Entry Programme.
If you are not a University Graduate you may also be able to apply via the Scottish Wider Access Programme here.
Aside from good grades what else do I need?
Having the right grades is just the start of the application process. Most Universities will need you to sit the University Clinical Aptitude Test (UCAT). UCAT is designed to test your attitudes and identify the professional behaviours required for new doctors and dentists to be successful in their clinical careers. You can try some sample tests here
I don’t think I can afford to study Medicine – is there funding available?
If you are resident in Scotland and study full-time in Scotland, the Student Awards Agency for Scotland (SAAS) should pay your tuition fees. For all enquiries relating to SAAS, please consult them directly.
SAAS student loans
If you are a Scottish student, you can apply for a SAAS student loan when you apply for tuition fee funding. Loan payments are paid monthly; and you should receive the first instalment within 3 or 4 days of registering as a student. Make sure you have enough money to support yourself for those first few days and please check whether the loan payment is in your account before spending money.
Grants
If you are eligible, SAAS can offer supplementary grants or a Young Student’s Bursary. Please consult SAAS for more information on grants.
Further information about SAAS eligibility, support available and how to apply can be found here:
Bursaries, Scholarships and Other Financial Support
You may also be able to apply for additional funding directly from your University. Use the links below to find our more about each universities arrangements:
Does a disability, Illness or mental health condition mean I can’t apply to study medicine?
A disability, chronic illness or mental health condition will not necessarily prevent you from becoming a doctor. The General Medical Council (independent regulator for doctors in the UK) states that “we firmly believe disabled people should be welcomed to the profession and valued for their contribution to patient care”.
You can also find out more about support available to you here Disability Guidance | Disabled Doctors Network.
Before you submit an application for medical school via UCAS, you should contact medical schools to request advice about your individual circumstances. Each medical school has a disability support adviser who can help.
All Universities offer confidential support services for disabled students.
This includes students with physical and sensory impairments, mental health difficulties and dyslexia.
You can find out more about what support is available by clicking the links below:
Do I need to pass an interview?
Yes, but you will be given guidance and support in advance of your interview and it’s designed to be a conversation rather than an a question and answer session.
While each medical school has its own interview process it is a vital part of the application and selection wherever you are applying. There are several sources of interview guidance online and it is worth practising with anyone you can – whether that is family, friends or teachers.
Remember that just like there is no “right” person for medicine, there is rarely a “right” answer in an interview. It is a conversation and your score will depend much less on what you say but more on how you say it. Try to have a reason behind any answer you give, make your answers as personal to your own experiences as you can and speak as clearly and confidently as you can. Enthusiasm and commitment are the key things an interviewer will be looking for.
The Royal College of Surgeons (England) has prepared a list of possible questions to help you prepare.
So why do you want to be a Doctor?
Source – Medic Insight Dundee
What’s it like being a medical student?
Once at university there are frequent assessments and you will have a more hectic schedule than most other students, but there is an immense camaraderie amongst fellow medical students and a sense of growing confidence in your own ability.
Each Medical School has a slightly different structure to their course, but broadly speaking the first couple of years are spent learning the basic science behind human physiology and disease, and the later years are spent learning how to apply this clinically. As you progress through medical school you will not only learn the knowledge to become a doctor but also the skills and attitudes you will need.
You will be taught by doctors, nurses and a whole range of other healthcare professionals and each one is committed to making you the best doctor you can possibly be.
After medical school you will progress through the different grades before qualifying as a Consultant or a General Practitioner – with competitive entry to each grade and post-graduate exams. These things should not put you off but it is important to be aware that the challenges continue long after university.
You can find out more about training to be a Doctor or visit the NHS Scottish Medical Training website.
Life as a Medical Student – Video Resources
Each student has their own experience of life at medical school but there are some things which are common to everyone. There is a huge feeling of being “in it together” and although there are regular challenges the support of your colleagues is always there. Most doctors still consider their time at medical school to be the most enjoyable time of their life.
Here is a selection of interviews with current medical students at Glasgow University to see how they feel about certain aspects of medical school.
Was this helpful?
Thinking of Studying Medicine?
Medicine is one of the most challenging but rewarding careers available. The combination of daily academic stimulation, technical procedural skills and working with patients, their families and the wider healthcare team is one which few other professions can offer.
Medicine is a profession that is open to everyone. There is no “right” person to be a doctor but all doctors are united by a passion for patient care and a dedication to their profession.
Working as a Doctor means you will train in and probably spend the majority of your career working within the National Health Service (NHS).
The NHS is Scotland’s largest single employer and one of the largest healthcare employers in the world. The Chief Executive of NHS Scotland heads the directorates and is accountable to ministers for the efficiency and performance of the service and the work of the 14 NHS Boards and 8 Special Health Boards.
The life of a doctor is not for everyone – long hours and witnessing distressing illness in patients at times can be stressful and emotionally demanding. But for those who are passionate about the profession the job satisfaction cannot be beaten.
If you are considering applying to study medicine we have created a number of resources you can access from the menu below to help you gain an insight into the career.
Please note that NHS Greater Glasgow and Clyde supports a number of programmes but is not responsible for content of an external website or involved in the selection of candidates for programmes.
___________________________________________________________________________________________________
So you have decided to study medicine…
___________________________________________________________
Main workstreams
____________________________________________________________________________________________________
Further Information
Was this helpful?
The Scottish Cytology Training School (SCTS) is a National Health Service Cervical Screening Programme (NHSCSP) Accredited Training Centre. The SCTS provides training and continuing professional development (CPD) for relevant professional staff in cervical cytology screening and associated work areas as part of the Scottish Cervical Screening Programme.
Scottish Cytology Training School Course Information
Please send completed application forms to: ggc.scts@nhs.scot
Scottish Cytology Training School Courses
Introductory Course in Gynaecological Cytology [NHSCSP Diploma] – (Thinprep®) – Minimum entry qualification
Trainee Cytoscreener – 4 GCSE’s
Trainee Biomedical Scientist – ‘A’ levels or equivalent to allow entry to a Health Profession Council (HPC) approved degree course or a recognised HPC/Institute of Biomedical Science (IBMS) approved degree.
Eligibility – All students must be employed in an NHSCSP Cytopathology department, as a trainee to undertake this course as part of the 2 year UK registration training.
Length of time in post: Learners should attend the introductory course ideally within the first 6 months of employment. Learners should spend a minimum of 6 weeks in the home laboratory learning how to set up as well as use a light microscope to visualise cells for interpretation and be familiar with normal cell morphology and basic infections.
NHSCSP – Registration: Prior to starting the introductory course pre-registration students must be registered by their employers with the NHSCSP Education office. The laboratory training officer (assessor) must also be registered prior to learners commencing their portfolio. This course is the first part of an intensive two year training plan for registration in Cytology which includes written portfolio work, slide logbook and attendance at compulsory courses at the training centre with a final one day external examination.
Follow –up Course in Gynaecological Cytology [NHSCSP Diploma] – (Thinprep®)
Course for candidates who have previously attended the NHSCSP Introductory Course in Gynaecological Cytology. This normally takes place between 6 to 12 months after the Introductory Course.
Pre-examination Course [NHSCSP Diploma] – (Thinprep®)
The introductory and follow up courses are supported by a pre-examination course. This normally takes places between 3 months and 3 weeks before the examination.
Biomedical Scientist (BMS)/Cytoscreener One Day Update Course
Update course to refresh qualified screeners knowledge and inform them about developments in Cervical Cytology and the NHSCSP.
Team Information



Find Us
Cytology Laboratory, Laboratory Medicine Building, Level 3,Queen Elizabeth University Hospital, 1345 Govan Road, Glasgow G51 4TF
Contact Numbers: 0141 354 9548 or 0141 354 9547

Transport & Travel Information: Transport, Travel and Parking – Information for Patients and Visitors
Training Resources
- Scottish Koilocyte Identification Project
- Useful Websites
- British Association of Cytology Updated Code of Practice 2022
- Review of Implementation of Recommendations into Cervical check screening programme
- Dr Amelia Trope Seminar – Cervical Screening in Norway Sept 25
- European Guidelines on Cervical Cancer Screening and Diagnosis
Was this helpful?
As part of our commitment to widening access to NHS employment we host a number of pre employment training programmes in partnership with the DWP and a number of employability agencies across Greater Glasgow and Clyde.
Our programmes provide training, work experience and application support to people experiencing barriers (real or perceived) to employment enabling them to become competitive job applicants.
Everyone who successfully completes our programmes will be guaranteed to be offered Job Interviews for suitable vacancies across our Health board area.
Project Search
The primary aim of Project SEARCH is to connect young people with learning disabilities and additional support needs with competitive employment. There are no formal entry requirements. However, applicants must participate in a selection process, where they may undertake assessments and interviews with a host business, and education partners, such as their school or local college to be accepted on Project SEARCH.
Interns are supported through placements within NHS Greater Glasgow and Clyde. They get hands-on experience and are given the opportunity to demonstrate their skills and abilities while learning new complex and varied, practical and vocational skills to help ready them for the world of work.
The NHSGGC programme is open to Glasgow City Council area residents and runs for one year, delivered in partnership with Glasgow Clyde College. Other Project Search opportunities are offered by City of Glasgow College
____________________________________________________________ Main workstreams
_________________________________________________________________________________________________________Further Information
Was this helpful?
There is a lot of information available on line to support you in planning your career pathway. These are just some of the resources you can access.
You can also contact Skills Development Scotland, Scotland’s National Skills Agency who offer specialist support to individuals to build their career management, work-based and employability skills, throughout their career journey, from school, into further learning opportunities and employment.
- NHS Scotland Careers Portal NHS Careers
- Information on Education and Training NES – NHS Education for Scotland Careers Information
- Information about NHS Careers My World of Work
- Turn your personal qualities into an NHS career My Career Options.
- How can I best advise my students? Information for Teachers and Careers Advisors
- How can I best support my child? Information for Parents/Carers – My World of Work and Information for Parents/Carers NHS Scotland Careers
- Skills Development Scotland Apprenticeships
- Young Person’s Guarantee
- Find out more about volunteering at Ready Scotland
- YouCanBeaDoctor – Information and Video resources for those considering Medicine as a career.
Was this helpful?
This section helps you get started, be more efficient with your computer or laptop, connect with patients, and use electronic patient records
Getting started
- If you are new to using digital in NHSGGC you will find lots of useful information in the GGC eHealth sharepoint site
- Whilst you will be trained on specific applications, consider a basic IT session to learn skills that span across applications such as file management, keyboard and mouse skills, and functions of Microsoft Windows.
- NHS Scotland uses Microsoft 365 (M365) which allows access to many useful apps. Training and information is available on the M365 Skills Hub.
- In particular, familiarise yourself with features of Outlook and Teams as the apps for communicating and collaborating.
- You can even set up your Teams profile with a photo. It is a national resource with 1000s of users so it can help identify yourself and others more quickly. Follow the steps on how to do this.
- Consider familiarising yourself with accessibility tools for you and your colleagues.
- Be sure to personalise your email and contact details in the global address list via eHelp ‘update contact details‘
Keeping safe
- The NHSGGC Information Security: Acceptable Use Policy gives a important background in the safe use of email, social media and use of devices. See Cyber Awareness for further information on simple ways to keep our information safe.
- Never share your logon details or passwords. Consider setting up Imprivata OneSign (Single Sign On) on your computer to help you to remember many of your passwords. It updates automatically each time you require to change a password.
- Always lock your computer when you step away from it even in an office setting. Use Windows key + L to lock the screen quickly.
Problem solving digital issues
- If an application is not working well, first consider if it is using an internet connection and if this connection is good. See Troubleshooting Network Problems. Most NHSGGC devices connect to ‘WPA2-MAIN’ in NHSGGC premises.
- If it is a problem with logging into a system, search for the ‘Forgot your password?’ or similar function. Make sure your set-up any security questions for systems you use before you need to use them in anger!
- Ask yourself what you expect the computer to be doing and what is now different?
- Make use of help functions in most applications, often depicted by a ?.
Also consider functions and settings often are in the ‘cog’, ellipsis (…), or other menu symbols. Remember that every symbol in an application means something, so hover over it with the mouse cursor, click it or right-click it to see what it does. - Consider asking ‘super-users’ in your team for tips and help.
- Use eHelp if the above hasn’t managed to solve your problem.
- Discuss the digital skills needed for you job in supervision, 1:1s and PDP&R just like you would for your clinical skills.
Home and agile working
- To work from home successfully you require a NHSGGC provided device (e.g. laptop) and a stable internet signal.
- To use M365 products such as Outlook and Teams, simply connect to your home Wi-Fi.
- To access clinical systems such as EMISWeb, Clinical Portal, TrakCare and Staffnet, you need to have a ‘secure’ connection. This could be achieved in several ways:
- By mobile connection where your device has a SIM card like you would have in a mobile phone. This is configured with some special settings that make the connection ‘secure’. This is the preferred option if you need access to these systems in a wide range of lcoations particularly patients’ homes.
- By connecting to your home Wi-Fi and then using a ‘remote connection’ tool such as F5. This can be requested through eHelp. This is the preferred option if you work from home for prolonged periods and tend to have numerous applications open at a time.
Electronic health and care records (EHCR)
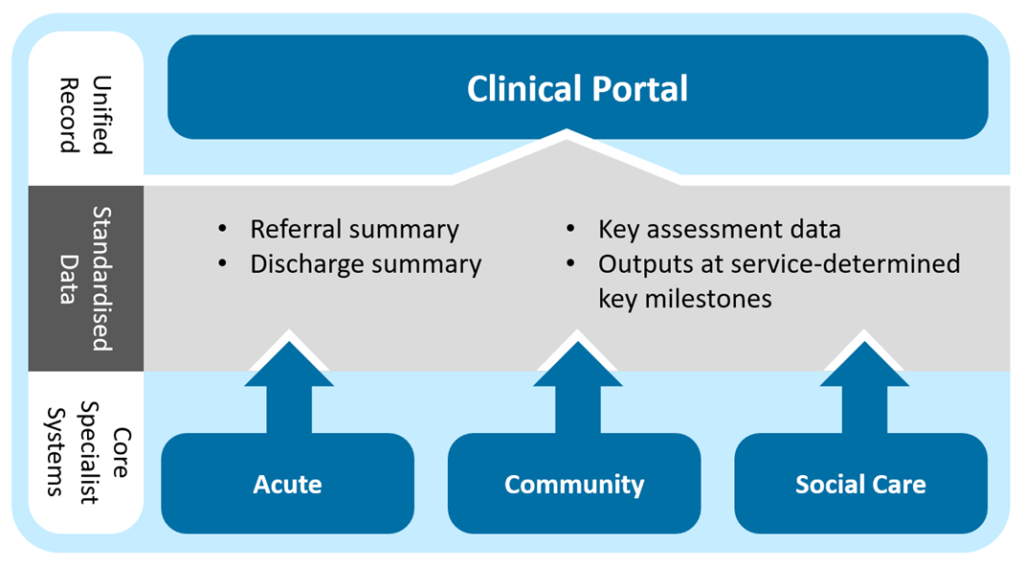
There are 3 ‘cornerstone’ systems used in NHSGGC:
- TrakCare used mainly in acute settings;
- EMISWeb used mainly in community settings; and
- Clinical Portal used across all settings including social care for viewing clinical information. Also used across a variety of settings for documenting information.
The diagram below illustrates the NHSGGC strategic plan of how they integrate:
Training
Connecting with patients
Connecting with our patients remotely is easy with Near Me. Speak with you manager to see if it is available in your service.
A recent patient feedback survey showed over 90% of patients that had used the video call service would use it again if offered. It has many benefits both to the patient and the clinician.
The Telerehab Toolkit is an excellent resource to help you feel more confident with video calls as well as some other really useful info and links.