Biochemistry
Click on an analyte name below for further information:
Adrenocorticotrophic Hormone (ACTH)
Adrenocorticotrophic hormone (ACTH) is a 39 amino acid peptide hormone secreted by the anterior pituitary, under the control of the hypothalamic peptide, corticotrophin-releasing hormone (CRH). ACTH secretion is pulsatile and exhibits diurnal variation, with highest plasma concentrations around 8am and lowest levels at midnight. It stimulates glucocorticoid (cortisol) production in the adrenal cortex.
ACTH measurement is only useful as a second line investigation following the finding of either cortisol deficiency or excess.
In cortisol deficiency due to primary adrenal failure, ACTH will be raised due to lack of negative feedback. ACTH will be low in adrenal insufficiency secondary to pituitary failure (hypopituitarism).
Excessive production of cortisol accompanied by suppressed ACTH may be seen in Cushing’s syndrome due to adrenal tumours/hyperplasia, and with exogenous glucocorticoid administration.
Cortisol excess with raised ACTH may be seen in ACTH-producing tumours of the pituitary (Cushing’s disease) or other tissues e.g. lungs (ectopic ACTH production).
NB. ACTH secretion may be increased by stress.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: EDTA
- Precautions: Separate and freeze plasma within 4 hours of sample collection. Transport frozen. Timing of collection important. Avoid stress. Haemolysed specimen unsuitable.
- Minimum Volume: 1 mL
- Reference Range: Not applicable
- Turnaround Time: 7 days
- Method: Siemens Immulite
- Quality Assurance: UK NEQAS
Anti-Mullerian Hormone (AMH)
Anti-Mullerian hormone (AMH) is a protein produced by granulosa cells of the ovaries in females and by Sertoli cells of the testes in males.
In women serum AMH concentration increases with age up until the mid-twenties, after which it begins to decline. AMH correlates well with the number of follicles in the ovary (as measured by ultrasound) in women over the age of 25 and its measurement is used to individualise fertility treatment.
In men serum AMH concentration tends to be high in childhood, then declines through puberty to low levels in adulthood. It is used in the investigation of cryptorchidism and anorchidism.
AMH is elevated in the majority of patients with granulosa cell tumours and may be used to monitor disease progression or recurrence. AMH is also useful in the investigation of disorders of sex development as a marker of testicular activity.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: Separate serum and send via first class post. If there will be >48 h before sending store the specimen at -20°C. Sample can be sent at ambient temperature.
- Minimum Volume: 2 mL
- Reference Range:
- Females: <50 pmol/L in young adults (falls steadily towards menopause where it becomes undetectable)
- Males (Levels fall at puberty. These ranges were derived from a study where stage of puberty was not determined):
- 0-1yr 390-1300 pmol/L
- 1-4yr 300-1700 pmol/L
- 5-8yr 260-1200 pmol/L
- 9-12yr 100-1000 pmol/L
- 13-16yr 40-560 pmol/L
- Adults <100 pmol/L (literature value)
- Turnaround Time: 14 days
- Method: Beckman Access
- Quality Assurance: UK NEQAS
Growth Hormone (GH)
Growth hormone (GH) is a peptide hormone secreted by the anterior pituitary. Its main action is to stimulate the production and release of insulin-like growth factor 1 (IGF-1) by the liver. Excessive secretion causes acromegaly, while deficiency causes failure of growth in children and metabolic problems in adults.
The secretion of GH is very episodic, so random measurement is rarely useful diagnostically.
Failure of GH to suppress during a glucose tolerance test is diagnostic for acromegaly.
Stimulation tests, such as an insulin tolerance test (NB. potentially dangerous, should only be carried out in centres experienced in it) or stimulation with arginine, GHRH/arginine or glucagon, can be carried out to test for insufficiency. GH deficiency may occur as part of a more general deficiency of pituitary hormones, so other hormones are often measured at the same time.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range:
- Random GH:
- > 10 μg/L excludes GH deficiency
- < 0.4 μg/L excludes acromegaly
- Severe Growth Hormone Deficiency:
- Adults Peak GH during ITT < 3 μg/L
- Adults Peak GH with GHRH/Arginine < 5 μg/L
- Children Peak GH during provocation < 5 μg/L
- GH Excess:
- Failure to suppress during OGTT < 1 μg/L
- Mean integrated 24hr GH > 1.7 μg/L
- Random GH:
- Turnaround Time: 7 days
- Method: IDS iSYS
- Quality Assurance: UK NEQAS
Insulin-like Growth Factor 1 (IGF-1)
Insulin-like growth factor 1 (IGF-1) is a peptide hormone, very similar to insulin. It is a major growth factor, which is synthesised by most cells and tissues. Circulating IGF-1 is produced by the liver in response to growth hormone (GH). IGF-1 concentration is increased in acromegaly, decreased in growth hormone deficiency and altered in systemic illness and malnutrition.
It is often measured along with growth hormone in the investigation of disorders of GH secretion. It is also used to monitor patients with acromegaly and those on growth hormone therapy.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range:
Age (yr) Males (μg/L) Females (μg/L)
<2 15 – 157 17 – 151
2 – 4 28 – 247 25 – 198
5 – 7 46 – 349 39 – 272
8 – 10 67 – 442 59 – 371
11 – 13 89 – 503 82 – 465
14 – 16 104 – 510 97 – 502
17 – 25 105 – 410 96 – 417
26 – 39 81 – 249 72 – 259
40 – 54 63 – 201 57 – 197
55 – 65 49 – 191 43 – 170
65+ 39 – 186 35 – 168
- Turnaround Time: 7 days
- Method: IDS iSYS
- Quality Assurance: UK NEQAS
Insulin
Insulin, produced by pancreatic beta cells, regulates glucose uptake and utilization and is involved in protein synthesis and triglyceride storage. It is often measured alongside C-peptide.
Clinical uses of insulin measurements:
- Evaluation of possible insulinoma: In cases of hypoglycaemia, diagnosis of insulinoma relies on proving inappropriate secretion of insulin during a hypoglycaemic episode.
- Hypoglycaemia of infancy due to hyperinsulinaemia.
- Diagnosis of factitious hypoglycaemia together with measurement of C-peptide.
- Discrimination of type 1 and type 2 diabetes mellitus: Insulin and C-peptide concentrations are generally low in patients with type 1 diabetes mellitus, and either normal or elevated in early type 2 diabetes, and decreased in later stages.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: Lithium heparin
- Precautions: Collect after overnight fast or during symptomatic hypoglycaemia, together with glucose sample. Separate and freeze plasma within 4 hours of sample collection. Transport frozen. Haemolysed specimens unsuitable. For hypoglycaemic screen, only measure when hypoglycaemic (glucose <2.6 mmol/L).
- Minimum Volume: 2 mL
- Reference Range: Not applicable
- Turnaround Time: 7 days
- Method: Abbott Alinity
- Quality Assurance: UK NEQAS
Insulin C-peptide
Insulin C-peptide (connecting peptide), a 31 amino acid polypeptide, represents the midportion of proinsulin. During insulin secretion it is enzymatically cleaved from proinsulin and co-secreted in equimolar proportion with mature insulin. The half life of C-peptide is significantly longer than insulin, so it is detectable in higher concentrations and the level less variable. C-peptide is often a more reliable marker than insulin. In addition, insulin is destroyed by proteases in haemolysed samples, while C-peptide is not.
Clinical uses:
- Insulinoma: Elevated C-peptide levels from increased beta-cell activity.
- Covert self-administration of insulin: Can be virtually ruled out as cause of hyperinsulinaemia by finding elevated C-peptide.
- Type 1 diabetes mellitus: Low C-peptide levels due to diminished insulin secretion, or suppressed as a normal response to exogenous insulin. Patients on insulin can develop anti-insulin antibodies which can interfere with insulin assay, so C-peptide can be used instead to check residual beta-cell activity.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: Lithium heparin
- Precautions: Collect after overnight fast. Separate and freeze plasma. Transport frozen.
- Minimum Volume: 1 mL
- Reference Range: Not applicable
- Turnaround Time: 7 days
- Method: Siemens Immulite
- Quality Assurance: UK NEQAS
Macroprolactin
Prolactin exists in various forms including the monomeric biologically active form (23kDa) and a higher molecular weight form, bound most commonly to IgG, known as macroprolactin (>100kDa). Macroprolactin lacks biological activity but can interfere in standard prolactin immunoassays and is a “common” cause of hyperprolactinaemia (overall prevalence 1.5%). Its presence is determined by recovery of prolactin following precipitation with polyethylene glycol (PEG screening test).
Macroprolactin should be requested in cases of persistently raised prolactin >700 mU/L (on two or more occasions) in euthyroid patients and after excluding drug associated hyperprolactinaemia. PEG screening can identify macroprolactin and determine the concentration of monomeric (bioactive) prolactin, as both may coincide.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range:
- Macroprolactin is reported as positive or negative based on percentage recovery of monomeric (bioactive) prolactin after PEG precipitation to remove macroprolactin:
- Post-PEG recovery <40% – macroprolactin detected
- Post-PEG recovery >60% – macroprolactin negative
- Post-PEG recovery 40 – 60% – equivocal recovery
- Macroprolactin is reported as positive or negative based on percentage recovery of monomeric (bioactive) prolactin after PEG precipitation to remove macroprolactin:
- Turnaround Time: 7 days
- Method: Polyethylene glycol (PEG) precipitation to precipitate macroprolactin followed by Abbott Alinity immunoanalyser to quantify monomeric prolactin.
- Quality Assurance: UK NEQAS
Parathyroid Hormone (PTH)
Parathyroid hormone (PTH), a polypeptide containing 84 amino acids, is secreted by the chief cells of the parathyroid glands. It has a molecular weight of 9.4 kDa. PTH should be measured in the investigation of unexplained hypercalcaemia or hypocalcaemia. PTH should always be interpreted in light of the serum adjusted calcium concentration and the patient’s renal function.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: EDTA
- Precautions: Avoid haemolysis
- Minimum Volume: 2 mL
- Reference Range: 1.6 – 7.5 pmol/L
- Turnaround Time: 1 day
- Method: Abbott Alinity
- Quality Assurance: UK NEQAS
Renin
Renin, a proteolytic enzyme, is synthesized by the juxtaglomerular cells of the kidney and released in response to decreased blood volume, decreased blood pressure and sodium depletion. Renin stimulates aldosterone release through angiotensin intermediates, resulting in the renal retention of sodium and the excretion of potassium.
Renin is measured with paired aldosterone to calculate an aldosterone/renin ratio in the investigation of hypertension.
Renin measurement may be useful in monitoring response to therapy in patients with Addison’s disease or congenital adrenal hyperplasia (CAH).
Beta blockers, diuretics, ACE inhibitors, angiotensin II receptor blockers, calcium channel blockers, a restricted salt diet and posture can all affect interpretation of renin results.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: EDTA (Lithium heparin unsuitable)
- Precautions: Do not collect on ice. Separate and freeze plasma within 4 hours of sample collection. Transport frozen. Grossly haemolysed or lipaemic samples unsuitable. Posture and relevant drug therapies (see above) may affect interpretation of results.
- Minimum Volume: 500 μL
- Reference Range:
- Adults (upright): <52 mIU/L
- Infants <1 year: <450 mIU/L
- Children 1 – 5 years: <380 mIU/L
- Children 6 – 15 years: <125 mIU/L
- Turnaround Time: 14 days
- Method: IDS iSYS
- Quality Assurance: UKNEQAS
Sex Hormone Binding Globulin (SHBG)
Sex hormone binding globulin (SHBG) is a large 80-100 kDa glycoprotein that functions to transport sex hormones around the body. It has a high affinity for 17β-hydroxy steroids such as testosterone and oestradiol. Concentrations of SHBG are influenced by many factors. SHBG will be increased by elevated concentrations of circulating oestrogens (including the oral contraceptive pill), hyperthyroidism, liver disease and excess alcohol. SHBG will be decreased by increasing body mass index, polycystic ovarian syndrome and hypothyroidism.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range:
Age Male (nmol/L) Female (nmol/L)
3 – 10 years 45 – 220 50 – 170
10 – 12 years 22 – 188 38 – 129
Adult 13 – 70 20 – 155
- Turnaround Time: 1 day
- Method: Abbott Alinity
- Quality Assurance: UK NEQAS
Anti-Thyroperoxidase (TPO) Antibodies
Anti-thyroperoxidase (TPO) antibodies are present in 90-100% of patients with Hashimoto’s thyroiditis, the commonest cause of autoimmune hypothyroidism. Anti-TPO is measured in patients with subclinical hypothyroidism (TSH 5-12 mIU/L and FT4 within reference limits: 8-21 pmol/L) to identify those at increased risk of developing thyroid failure. The risk of developing hypothyroidism if anti-TPO is positive is approximately 5% per year.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range: <6 IU/L
- Turnaround Time: 1 day
- Method: Abbott Alinity
- Quality Assurance: UK NEQAS
TSH Receptor Antibody (TRAB)
TSH receptor antibody (TRAB) is measured if the cause of thyrotoxicosis is not clear. It is specific for Graves’ autoimmune disease of thyroid but is not present in all cases. It can be used to distinguish Graves’ disease from toxic multinodular goitre and postpartum or subacute thyroiditis. It is also measured in 3rd trimester of pregnancy, if there is a maternal history of Graves’ disease/thyrotoxicosis, to predict risk of neonatal thyroid problems. It may be helpful in cases of possible “euthyroid” Graves’ ophthalmopathy.
Sample Requirements and Reference Ranges
- Sample Type: Serum (plasma unsuitable)
- Container: SST
- Precautions: Grossly lipaemic samples unsuitable
- Minimum Volume: 2 mL (Neonatal samples: minimum serum volume 200 µL)
- Reference Range:
- <3.1 U/L – negative
- ≥3.1 U/L – positive
- Turnaround Time: 14 days
- Method: Abbott Alinity
- Quality Assurance: UK NEQAS
Laboratory Sites and Contact Details
Glasgow Royal Infirmary (GRI)
The laboratory is located in the MacEwen Building on Alexandra Parade (adjacent to A and E). It provides routine service Monday to Friday between 9.00am and 5.00pm and on Saturday between 9.00am and 12.00pm. An emergency service operates at all times.
Gartnavel General Hospital (GGH)
The laboratory is located in the Laboratory Block on Shelley Road and operates Monday to Friday between 8.45am and 9.00pm. NB. Samples are only accepted into the GGH lab until 7.30pm. After this time, samples are directed to the porters’ box and transported to GRI for analysis.
New Stobhill Hospital
A small satellite laboratory is located on Level 1 and operates Monday to Friday between 9.00am and 5.00pm.
Enquiries (9.00am until 5.00pm)
There is a central reporting office located at GRI which covers the three North Glasgow laboratory sites.
Duty Biochemist/General Enquiries 0141 242 9500 (x.29500)
Enquiries (Out of Hours)
Contact the on-call biochemist via switchboard 0141 211 4000
Emergency Laboratories (24/7)
Glasgow Royal Infirmary call 0141 211 4487 (x.24487)
Accreditation and Quality
North Glasgow Biochemistry is a medical testing laboratory accredited to ISO 15189:2012 by the United Kingdom Accreditation Service (UKAS). Our UKAS Medical Accreditation number is 9572. A full list of accredited tests can be found on our schedule of accreditation. Tests not on this list are not accredited; please contact the laboratory for further information if required.
The laboratory participates in external quality assurance schemes where available. Performance details are available upon request. If you wish to provide feedback on the North Glasgow Biochemistry service, please contact our Quality Manager.
The Biochemistry department utilises the Telepath Laboratory Information Management System (LIMS) and TrakCare. Due to the limitations of this software, we are currently unable to fully meet the requirements of the UKAS publication GEN-6 – Reference to accreditation and multilateral recognition signatory status.
This publication sets out the requirements of reports/results released by the laboratory to contain the appropriate use of UKAS logos and identify any tests that are accredited and those that are not. The department have risk assessed this. Due to the number of analytes that can be listed on a Biochemistry report, the number of tests that are UKAS accredited and the number of auto comments already added, it is agreed by the laboratory management team that an additional auto comment would detract from the clinically relevant comments and potentially could push these onto a second page where they may be missed altogether. The risk is magnified by the way TrakCare displays results, as any result with a comment has an icon displayed next to it. If an icon is displayed next to almost every result, the alert loses its impact and may lead to clinicians missing critical icons and comments.
Although we are not able to present this information on our reports, the department’s user’s handbook and website provide full details of our accreditation.
Laboratory Handbooks
Forms
During periods of Trakcare downtime, Biochemistry requests must be made on paper request forms. The paper request form should also be used for add on requests. Request forms can be ordered through PECOS, however a pdf copy of the Biochemistry is available for download and printing.
Laboratory Newsletters
Clinical Audit and User Survey Reports
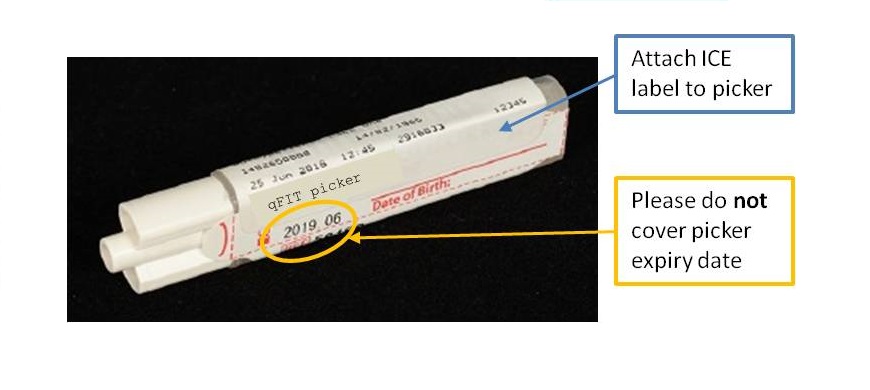
Since Monday 3rd September 2018, quantitative faecal immunochemical testing (qFIT) has been available to Primary Care in NHS Greater Glasgow and Clyde. The service is provided by the Biochemistry department in Glasgow Royal Infirmary. qFIT is designed to detect small amounts of blood in stool samples using antibodies specific to human haemoglobin. Analysis is carried out on the HM-JACKarc system (Alpha Laboratories), with a positive result being 10 µg Hb/g faeces and above.
The NHSGGC Colorectal Cancer Referral Guidance has the latest guidelines and information, along with qFIT patient information leaflets in a number of languages.
Lab Handbooks and Manuals, previous editions of the laboratory newsletter and older memos are available below:
Laboratory Handbooks and Manuals
Laboratory Newsletters
- Newsletter 1 – August 2014
- Newsletter 2 – November 2014
- Newsletter 3 – April 2015
- Newsletter 4 – September 2015
- Newsletter 5 – March 2016
- Newsletter 6 – August 2016
- Newsletter 7 – February 2017
- Newsletter 8 – October 2017
- Newsletter 9 – December 2017
- Newsletter 10 – April 2018
- Newsletter 11 – October 2018
- Newsletter Supplemental – QFIT
- Newsletter 12 – February 2019
- Newsletter 13 – July 2019
- Newsletter 14 – December 2019
- Newsletter 15 – February 2020
- Newsletter 16 – July 2020
- Newsletter 17 – December 2020
- Newsletter 18 – October 2021
- Newsletter 19 – March 2022
User Satisfaction Surveys
- Primary Care 2017 (2022 coming soon)
- Secondary Care 2021
Memos (Primary Care)
- Patient identification for patients without CHI (Dec 2018)
- The importance of putting samples in the correct bag for transport to laboratories (Dec 2018)
- Transport bags poster (Dec 2018)
- Transport bags poster (Bute and Dunoon) (Dec 2018)
- Please do not send requests for tests done by other disciplines on Biochemistry / Haematology request forms (Jan 2020)
- QFit for practices not using ICE electronic ordering (Jan 2020)
- Changes to the urine catecholamine and 5HIAA service (GRI memo, Mar 2020)
Memos (Secondary Care)
- Use of paper request forms when Trakcare not available (Nov 2018)
- Sending samples requested on Trakcare to the laboratory (Nov 2018)
- Cryoglobulin service at Vale of Leven (Nov 2018)
- Barcodes for Point of Care Testing (Nov 2018)
- Urine pregnancy test false positive results (Jul 2019)
- Biochemistry samples apparently being put in Microbiology bags (Aug 2019)
- New P3NP assay (Oct 2019)
- GEM 5000 gas analysers (Nov 19)
- Biochemistry samples apparently being put in Microbiology or Virology bags (Apr 2020)
The South Glasgow Biochemistry Service comprises a main Clinical Biochemistry Laboratory at the Queen Elizabeth University Hospital campus (QEUH and Royal Hospital for Children, RHC) along with a satellite blood science laboratory at the Victoria ACH. The Biochemistry service operates from the new QEUH Laboratory Building and Specialist Metabolic work and Toxicology Services for NHSGGC have centralised to this site.
South Glasgow Biochemistry services are accredited by the United Kingdom Accreditation Service (UKAS). UKAS Medical Accreditation number is 9569 (accredited to ISO 15189:2012), issue date 21st January 2022 and our certificate of accreditation is available to view.
A full list of tests in scope can be found on our schedule of accreditation. Tests not on this list are not accredited; please contact the laboratory for further information if required. Upon sending samples to the laboratory, please refer to our terms and conditions.
We are committed to providing a quality service to users. We welcome feedback and survey users on an annual basis to assess satisfaction with the service and highlight possible areas for improvement. For feedback on our service, please contact our Quality Manager, contact details for senior staff can be found in the laboratory handbook (below).
Latest News
- New National Guidance on GP requesting for certain laboratory tests
- During periods of TrakCare downtime, Biochemistry requests need to be made on a paper request form. These can be ordered through PECOS and a pdf copy is available for download and printing
- All Blood Gas analysers (GEM 5000) are now located solely within clinical areas within the main hospital campus, positioned according to current clinical need. A list of all locations is available at each blood gas analyser. We do not have the facility to carry out Blood Gas analysis within the laboratory
Contact Information
Telephone
The Main laboratory contact number is 0141 354 9060 (89060 for use within the hospital). An auto-attendant system is in operation to route your call more effectively. Please listen carefully to the new message which will direct you as follow:
- All results and add on tests, press 1
- Information on all sample requirements, press 2
- For advice on Blood Gas or Blood Glucose analysers, press 3
- For interpretation of results, clinical advice and emergency requests, press 4
In addition to the telephone options there are two email accounts which will be answered Monday to Friday 9.00am – 5.00pm, which are available for specific, non-urgent requests, processed at the QEUH:
- SouthGlasgow.BiochemistryAddOn@ggc.scot.nhs.uk
- for NON-urgent add on tests for Biochemistry samples sent to the South Glasgow Biochemistry laboratory only.
- You must include the following information in your email : CHI Number/DOB, surname, Date and Time of original sample, original location of sample, Test/Analysis to be added on.
- ggc.qeuhbiochemistsggc@nhs.scot – this is an email for Non-Urgent Clinical Advice
- Please include your query as well as the full patient details
- SouthGlasgow.BiochemistryPOCT@ggc.scot.nhs.uk
- for NON- urgent enquiries about point of care Blood Gas and Blood Glucose systems based in the QEUH, RHC, New Victoria, Mearnskirk and Leverndale Hospital
Our address
Department of Biochemistry,
Level 1, Laboratory Medicine and Facilities Management Building,
Queen Elizabeth University Hospital,
1345 Govan Road,
Glasgow
G51 4TF
Laboratory Opening Hours
The main service hours are 9am to 5pm Monday to Friday, 9.00am to 12.30pm Saturday, Sunday and Public holidays (9am to 5pm Monday to Friday only for satellite Blood Sciences Lab at New Victoria ACH). Outwith these hours a reduced analytical service is provided.
Out of Hours Service
For requests outwith the working day, a limited repertoire of urgent analyses can be undertaken as an emergency.
The BMS on duty can be contacted on Page 17684 (QEUH).
A consultant is always available for advice.
Outside working hours he/she may be contacted via the hospital switchboard.
Service Handbooks and Reference Ranges
In our Laboratory Handbook you will find comprehensive information regarding the use of the Biochemistry service, including information on test repertoire, specimen requirements, urgent requests and details of specialist assays available. Special advice on tests/investigations can also be found within our Metabolic Handbook. GP users across GG&C can find additional guidance in the General Practice Handbook.
We provide comprehensive reference ranges for our tests. These are available when accessing results via Trakcare and Clinical portal/SCI store and are also printed on Biochemistry Report forms. Information on reference ranges is also provided within our Laboratory Handbook. Interpretative comments are also provided on reports, where appropriate. For more detailed reference range data and for further interpretation of results, please contact the Department. Reference ranges will be regularly reviewed and amended as required.
Other Guidance
- Information on the services provided by the Toxicology Service at the QEUH
- Additional guidance on Tumour markers (guidance for the non specialist on the appropriate use of commonly requested tumour markers) is now available in the form of a ‘tumour marker bookmark‘. This has been recently released by the Scottish Clinical Biochemistry Network (SCBN), with the backing of the Royal College of Pathologists and the Realistic Medicine Program.
- 1
- 2
The Scottish Clinical Biochemistry Network has published a Tumour Marker Requesting Bookmark which provides guidance on the appropriate use of commonly requested serum tumour markers.
We have recently carried out an Audit of CEA requesting in Primary Care across NHSGGC.
Click on an analyte name below for further information:
Alpha-Fetoprotein (AFP)
Alpha-fetoprotein (AFP) is a 591 amino acid glycoprotein produced by the liver and yolk sac of a developing baby during pregnancy. Plasma concentrations begin decreasing at the end of the first trimester of pregnancy and fall rapidly after birth, with normal adult concentrations achieved by the age of 8 to 12 months. AFP is increased in neonates, during pregnancy and in some patients with benign liver disease. AFP is usually measured to assist with diagnosis of primary hepatocellular carcinoma or non seminomatous germ cell tumour (NSGCT) and to monitor treatment or detect recurrent disease in patients with these tumours. N.B. Send AFP for maternal screening to Medical Genetics, QEUH.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range: ≤6 kU/L
- Turnaround Time: 1 day
- Method: Abbott Architect/Alinity
- Quality Assurance: UK NEQAS
Carbohydrate Antigen 125 (CA125)
CA125 (carbohydrate antigen 125) is a glycoprotein MW >200 kDa. It is present in tissues derived from the foetal coelomic epithelium. In adults it occurs on the pleura and peritoneum, the gastrointestinal tract and female reproductive tract, including the endometrium. CA125 may be increased in women at the time of menstruation, in endometriosis, benign ovarian disease and renal or liver disease, and may be very high in early pregnancy. It is also elevated in patients with ascites, a pleural effusion or CCF. Measurement is usually restricted to monitoring the treatment of ovarian carcinoma.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range: ≤35 kU/L
- Turnaround Time: 1 day
- Method: Abbott Architect/Alinity
- Quality Assurance: UK NEQAS
Calcitonin
Calcitonin is a 32 amino acid peptide synthesised, stored and secreted by the C-cells of the thyroid. Measurement of calcitonin is useful in the diagnosis and monitoring of medullary thyroid carcinoma (MTC), a rare tumour, accounting for only 10% of all thyroid carcinomas. MTC can occur as a sporadic tumour or inherited as part of multiple endocrine neoplasia type 2 (MEN 2). C-cell hyperplasia can increase calcitonin concentration and response to calcium infusion can be used to distinguish hyperplasia from MTC. NB. Calcitonin is only useful as a screening test in patients where there is a known family history of MTC.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: Lithium heparin
- Precautions: Separate and freeze plasma within 4 hours of sample collection. Transport frozen.
- Minimum Volume: 1 mL
- Reference Range: <9 ng/L
- Turnaround Time: 14 days
- Method: Siemens Immulite
- Quality Assurance: UK NEQAS
Carcinoembryonic Antigen (CEA)
Carcinoembryonic antigen (CEA) is a highly glycosylated cell surface glycoprotein involved in intercellular adhesion. Its size varies in different organs from 90 to 200 kDa, due to variable glycosylation. CEA may be elevated in smokers and a number of benign liver, renal, lung or gastrointestinal tract conditions. Therefore CEA is not a useful screening or diagnostic test. Its main role is to monitor response to therapy and detect recurrent gastrointestinal malignancy.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2mL
- Reference Range: ≤5 μg/L
- Turnaround Time: 1 day
- Method: Abbott Architect/Alinity
- Quality Assurance: UK NEQAS
Chromogranin A
Chromogranin A is an acidic 439 amino acid glycoprotein (48 kDa) originating from the chromaffin granules of most neuroendocrine cell types. In health, chromogranin A is released as a pro-hormone together with other peptide hormones in response to stimulation. Larger quantities of chromogranin A are produced by neuroendocrine derived tumours thus allowing it to be used as a tumour marker. Chromogranin A is the most commonly raised neuroendocrine tumour (NET) marker.
This guidance details which tumour markers to request during the diagnosis and monitoring of NETs.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST/Plain Serum
- Precautions: Spin and separate within 8 hours. Store serum for up to four days refrigerated. Store frozen if longer term storage is required. Samples from referral laboratories can be posted at room temperature using 1st class post.
- Minimum Volume: 1 mL
- Reference Range: <95 µg/L
- Turnaround Time: 28 days
- Method: CisBio ELISA
- Quality Assurance: UK NEQAS
Gastrin
Gastrin-secreting cells (G-cells) produce, store and release gastrin within the pyloric and upper duodenal mucosa. Gastrin stimulates gastric acid secretion by parietal cells and circulates in 2 active forms: gastrin-34 (G-34) and gastrin-17 (G-17). Determination of circulating gastrin concentrations can aid in the diagnosis of gastrinoma. Greater than 50% are malignant and approximately 25% occur as part of multiple endocrine neoplasia type 1 (MEN 1). The presence of gastrinoma and hypergastrinaemia resulting in severe refractory peptic ulcer disease is known as Zollinger-Ellison syndrome. Increased circulating gastrin concentrations can also occur as a result of reduced or absent gastric acid secretion e.g. H pylori infection, chronic atrophic gastritis +/- pernicious anaemia or long-term use of proton pump inhibitors (PPIs). This is due to the lack of inhibitory feedback of acid on the G-cells. Therefore elevated gastrin levels should be interpreted in relation to gastric acid secretion.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: Heparinised plasma (EDTA unsuitable)
- Precautions: Sample should be collected after an overnight fast. Separate and freeze plasma within 4 hours of sample collection. Transport frozen. Proton pump inhibitors should be discontinued for a week, and H2 blockers for 48 hours, prior to sampling. Icterus and lipaemia moderately reduce results.
- Minimum Volume: 1 mL
- Reference Range: <115 µg/L (fasting)
- Turnaround Time: 28 days
- Method: Siemens Immulite. This assay measures both the G-17 isoform and, to a lesser extent, the G-34 isoform.
- Quality Assurance: UK NEQAS
Human Chorionic Gonadotrophin (HCG)
Human chorionic gonadotrophin (HCG) is a glycoprotein hormone produced by trophoblastic tissue. This is found in the placenta in normal pregnancy, in choriocarcinoma and in trophoblastic elements in germ cell tumours. HCG consists of two subunits (alpha and beta). When HCG is used as a tumour marker it is important that both free beta subunit and intact HCG are measured. HCG is used to diagnose, monitor or detect recurrent disease in germ cell tumours.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range: As tumour marker ≤5 U/L
- Turnaround Time: 1 day
- Method: Abbott Architect/Alinity
- Quality Assurance: UK NEQAS
5-Hydroxyindole Acetic Acid (5-HIAA) (Urine)
Metastatic carcinoid tumours arising from enterochromaffin cells produce excessive amounts of serotonin. The main metabolite of serotonin, 5-hydroxyindole acetic acid (5-HIAA) is excreted in urine and its measurement can be used to diagnose and monitor carcinoid tumours.
Sample Requirements and Reference Ranges
- Sample Type: 24 hr urine (acidified)
- Container: 24 hr urine container with 50 mL hydrochloric acid
- Precautions: Elevated by dietary walnuts, bananas, tomatoes, avocado, kiwi fruit, pineapple, plantain, plums, pecan nuts. Avoid for 3-4 days prior to starting urine collection. Patient information sheet for urine collection
- Minimum Volume: 10 mL
- Reference Range: ≤ 42 μmol/24 h
- Turnaround Time: 14 days
- Method: Liquid chromatography-tandem mass spectrometry
- Quality Assurance: UK NEQAS
Metadrenalines (Plasma)
The plasma free metadrenalines profile:
- Metadrenaline
- Normetadrenaline
- 3-methoxytyramine
Plasma free metadrenalines are used for the diagnosis of catecholamine producing tumours including phaeochromocytoma or paraganglioma (PPGL). Patients with PPGL may present with episodes of hypertension with palpitations, severe headaches and sweating. Patients may be asymptomatic but have an incidentally discovered adrenal mass.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: EDTA
- Precautions: Plasma must be separated from red blood cells within 2 hours of collection. Certain medications may cause false elevations of plasma metadrenalines. If clinically feasible, it is optimal to discontinue these medications at least 1 week before sample collection.
- Minimum Volume: 500 µL
- Reference Range:
- Metadrenaline < 510 pmol/L
- Normetadrenaline < 1180 pmol/L
- 3-methoxytyramine < 180 pmol/L
- Turnaround Time: 14 days
- Method: Liquid chromatography-tandem mass spectrometry
- Quality Assurance: UKNEQAS
Metadrenalines (Urine)
The urine free metadrenaline profile:
- Metadrenaline
- Normetadrenaline
- 3-methoxytyramine
Urine free metadrenalines are used for the diagnosis of catecholamine producing tumours including phaeochromocytoma or paraganglioma (PPGL). Patients with PPGL may present with episodes of hypertension with palpitations, severe headaches and sweating. Patients may be asymptomatic but have an incidentally discovered adrenal mass.
Sample Requirements and Reference Ranges
- Sample Type: 24hr urine
- Container: Plain urine container (no preservative)
- Precautions: Certain medications may cause false elevations of urine metadrenalines. If clinically feasible, it is optimal to discontinue these medications at least 1 week before sample collection. Patient information sheet for urine collection
- Minimum Volume: 10 mL
- Reference Range:
- Metadrenaline < 350 nmol/24 h
- Normetadrenaline < 650 nmol/24 h
- 3-methoxytyramine < 400 nmol/24 h
- Turnaround Time: 14 days
- Method: Liquid chromatography-tandem mass spectrometry
- Quality Assurance: RCPA
Prostate Specific Antigen (PSA)
Prostate specific antigen (PSA), a protease, is a normal constituent of seminal fluid. It is produced by the secretory cells of the acini and ducts of the prostate and other cells expressing the nuclear androgen receptor. PSA is measured to aid the diagnosis and for monitoring of prostate cancer. PSA may be spuriously elevated in a number of situations: catheterisation; acute retention of urine; urinary infection; ejaculation or vigorous exercise; DRE; prostate biopsy. Where these may have contributed to an elevated PSA result, suggest repeat in 6 weeks.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range:
- Age (yrs) PSA (μg/L)
- <60 ≤ 3.0
- 60 – 69 ≤ 4.0
- ≥70 ≤ 5.0
- Turnaround Time: 1 day
- Method: Abbott Architect/Alinity
- Quality Assurance: UK NEQAS
Thyroglobulin and Thyroglobulin Antibodies
Thyroglobulin (Tg), a protein produced by normal or malignant thyroid tissue, is used to monitor treatment of differentiated thyroid cancer and to detect recurrence. Tg measured a few months after total thyroidectomy for thyroid carcinoma provides valuable prognostic information. Please note that recent thyroid biopsy or surgery will cause an increase in Tg. 15-20% of thyroid cancer patients have thyroglobulin antibodies (TgAb). If present, TgAb can interfere with Tg measurements causing an artefactually low result. TgAb status may alter during treatment and TgAb should therefore always be measured on all Tg samples. Persistent or increasing concentrations of TgAb following thyroidectomy may indicate residual or recurrent tumour. Tg measurement is also of use in establishing the presence or absence of thyroid tissue in neonates with congenital hypothyroidism.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: Plain/SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range: Not applicable
- Turnaround Time: 7 days
- Method: Beckman Access
- Quality Assurance: UK NEQAS
We provide specialist Endocrine services for all Scottish Health Boards. Working collaboratively, we are actively involved in research and development projects and clinical audit.
Contact Information
NHSGGC Specialist Endocrine Laboratory
- General enquiries: 0141 242 9500, option 2
Contact Telephones
- Karen Smith, Consultant Clinical Scientist: 0141 201 6434
- Dr Maurizio Panarelli, Consultant Clinical Biochemist: 0141 242 9573
- Donna Chantler, Principal Clinical Scientist: 0141 242 9525
- Alison Fairservice, Principal Clinical Scientist: 0141 242 9520
- Amy Frank, Principal Clinical Scientist (USP): 0141 242 9534
- Susan Johnston, Principal Clinical Scientist: 0141 242 9537
Address
Department of Clinical Biochemistry
Macewen Building
Glasgow Royal Infirmary
Glasgow
G4 0SF
Accreditation and Quality
North Glasgow Biochemistry is a medical testing laboratory accredited to ISO 15189:2012 by the United Kingdom Accreditation Service (UKAS). Our UKAS Medical Accreditation number is 9572. A full list of accredited tests can be found on our schedule of accreditation. Tests not on this list are not accredited; please contact the laboratory for further information if required.
The laboratory participates in external quality assurance schemes where available. Performance details are available upon request. If you wish to provide feedback on the North Glasgow Biochemistry service, please contact our Quality Manager.
The Biochemistry department utilises the Telepath Laboratory Information Management System (LIMS) and TrakCare. Due to the limitations of this software, we are currently unable to fully meet the requirements of the UKAS publication GEN-6 – Reference to accreditation and multilateral recognition signatory status.
This publication sets out the requirements of reports/results released by the laboratory to contain the appropriate use of UKAS logos and identify any tests that are accredited and those that are not. The department have risk assessed this. Due to the number of analytes that can be listed on a Biochemistry report, the number of tests that are UKAS accredited and the number of auto comments already added, it is agreed by the laboratory management team that an additional auto comment would detract from the clinically relevant comments and potentially could push these onto a second page where they may be missed altogether. The risk is magnified by the way TrakCare displays results, as any result with a comment has an icon displayed next to it. If an icon is displayed next to almost every result, the alert loses its impact and may lead to clinicians missing critical icons and comments.
Although we are not able to present this information on our reports, the department’s user’s handbook and website provide full details of our accreditation.
Endocrine Tests
Click on an analyte name below for further information:
Aldosterone
Aldosterone is produced in the zona glomerulosa of the adrenal glands in response to renin and angiotensin intermediates. Measurement of aldosterone is most useful in the investigation of hypertension when measured concurrently with renin so that an aldosterone/renin ratio may be calculated.
Beta blockers, diuretics, ACE inhibitors, angiotensin II receptor blockers, calcium channel blockers, a restricted salt diet and posture can affect interpretation of aldosterone results.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: EDTA
- Precautions: Posture and relevant drug therapies (see above) may affect interpretation of results.
- Minimum Volume: 1.5 mL
- Reference Range:
- Adults (upright): 130 – 800 pmol/L
- Neonates <1 month: 1000 – 5500 pmol/L
- Infants (1-6 months): 500 – 4500 pmol/L
- Infants (6-12 months): 160 – 3000 pmol/L
- Children (2-4 years): 130 – 1000 pmol/L
- Children (5-15 years): 130 – 600 pmol/L
- Turnaround Time: 14 days
- Method: IDS iSYS
- Quality Assurance: UK NEQAS
Bloodspot 17-hydroxyprogesterone (17OHP)
17-hydroxyprogesterone (17OHP) is one of the intermediary steroid metabolites in the cortisol biosynthetic pathway. The most common genetic defect in cortisol production is deficiency of the 21-hydroxlase enzyme, which leads to congenital adrenal hyperplasia (CAH). 17OHP concentrations are raised in this form of CAH (approximately 90% of CAH cases) and is a useful marker to monitor response to therapy. Measuring 17OHP in blood spot samples is less invasive than venepuncture and allows multiple samples to be taken over a 24hr period.
Blood spot 17OHP is not a diagnostic test and is only useful in monitoring treatment.
Sample Requirements and Reference Ranges
- Sample Type: Whole blood spotted onto pre-prepared card (available on request)
- Container: N/A
- Precautions: None
- Minimum Volume: Ensure that blood soaks through to the back of the card
- Reference Range: N/A
- Turnaround Time: 56 days
- Method: Liquid chromatography-tandem mass spectrometry
- Quality Assurance: RfB
Dehydroepiandrosterone sulphate (DHEAS)
Dehydroepiandrosterone sulphate (DHEAS) is the sulphated ester of the 19-carbon androgen DHEA, produced by the adrenal gland. DHEAS is the most abundant circulating androgen and shows no diurnal rhythm. DHEAS acts as a precursor to other androgens, such as androstenedione and testosterone.
Measurement of DHEAS may be of benefit for the investigation of excess androgen. DHEAS is relatively specific for the adrenal glands, whereas other androgens, such as testosterone and androstenedione are also produced by the gonads.
Measurement of DHEAS is unhelpful in adult males.
Sample Requirements and Reference Ranges
- Sample Type: Serum or Plasma
- Container: SST or Lithium Heparin
- Precautions: None
- Minimum Volume: 500 μL (140 μL for neonates)
- Reference Range:
- Pre-pubertal: <2.0 μmol/L
- Adult female <50 yr: ≤9.6 μmol/L
- Adult female ≥50 yr: ≤3.1 μmol/L
- Turnaround Time: 14 days
- Method: Liquid chromatography-tandem mass spectrometry
- Quality Assurance: UK NEQAS
Salivary Cortisol
Cortisol is an essential glucocorticoid steroid produced by the adrenal cortex. Cortisol circulates bound to cortisol binding protein (CBG) with only 15% being the unbound biologically active form. The saliva concentration generally reflects the free cortisol concentration in serum and may be useful in the investigation of cyclical Cushing’s syndrome due to the non-invasive nature of sample collection.
Sample Requirements and Reference Ranges
- Sample Type: Saliva (passive drool)
- Container: 5 mL plain (can be supplied by laboratory)
- Precautions: If multiple samples collected over several weeks, store frozen and send by 1st class post.
- Minimum Volume: 2.5 mL
- Reference Range:
- am: <20 nmol/L
- pm: <5 nmol/L
- Turnaround Time: 35 days
- Method: Liquid chromatography-tandem mass spectrometry
- Quality Assurance: UKNEQAS
Serum Androgen Profile
The serum androgen profile simultaneously measures:
- testosterone
- androstenedione
- 17-hydroxyprogesterone (17OHP)
- 11-deoxycortisol (11DOC)
- 21-deoxycortisol (21DOC)
11DOC and 21DOC are not routinely reported. If an abnormality is detected in either, a comment will be made on the report.
The androgen profile is recommended for investigation of hirsutism, polycystic ovarian syndrome (PCOS) and infertility in females, and for the diagnosis and monitoring of congenital adrenal hyperplasia (CAH) in both males and females. Please state clinical details and menstrual cycle information on the request form.
Androgens pre- and 60-min post synacthen may be of benefit for the investigation of late onset CAH if elevated androgens have been observed in a follicular phase sample.
In neonates, 17OHP can be measured from the day of birth for the investigation of CAH, however levels may continue to rise immediately after birth, with further adrenal stimulation. An elevated 21DOC would confirm 21-hydroxylase deficiency CAH.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST. Please send primary sample if possible. Some interference has been observed with certain aliquoter tubes, such as the Impeco tube.
- Precautions: None
- Minimum Volume: 500 μL (140 μL for neonates)
- Reference Range:
- Adult Females:
- Testosterone <1.5 nmol/L
- 17-Hydroxyprogesterone <6.0 nmo/L
- Androstenedione (18 – 40yrs) <5.5 nmol/L
- Androstenedione (>40yrs) <3.0 nmol/L
- Adult Males:
- Testosterone 7.0 – 30 nmol/L
- 17-Hydroxyprogesterone <6.0 nmol/L
- Androstenedione <5.5 nmol/L
- Paediatric ranges under evaluation
- Adult Females:
- Turnaround Time: 7 days (Please contact the lab to notify of any urgent neonatal sample)
- Method: Liquid chromatography-tandem mass spectrometry
- Quality Assurance: UK NEQAS
Testosterone
Testosterone is a 19-carbon androgen, produced by both the adrenal glands and gonads. Production is controlled by LH or HCG. Serum testosterone is often measured in female patients to investigate suspected polycystic ovary syndrome (PCOS) or idiopathic hirsutism. However, some women will have a more serious pathology, such as adrenal/ovarian tumours, Cushing’s syndrome or late onset congenital adrenal hyperplasia (CAH).
In females, testosterone, androstenedione and 17-hydroxyprogesterone (17OHP) are lowest in the follicular phase. In males, testosterone is highest early in the morning and declines through the day.
Androstenedione
Androstenedione is a 19-carbon androgen, produced by both the adrenal gland (ACTH control) and gonads (LH or HCG control) and also by peripheral conversion from testosterone. Androstenedione has 20% of the androgenic potency of testosterone.
Androstenedione is most commonly measured in women for the investigation of polycystic ovarian syndrome (PCOS).
Androstenedione may be helpful in disorders of puberty. It is raised in cases of congenital adrenal hyperplasia (CAH) due to deficiency of the 21- or 11β-hydroxylase enzymes and may be useful in the diagnosis of these conditions and in the monitoring of glucocorticoid replacement therapy. Androgen secreting tumours of both the adrenal (adenoma and carcinoma) and ovary (arrhenoblastoma, hilar cell and granulosa cell) may result in high serum levels of androstenedione.
17-Hydroprogesterone (17OHP)
17-hydroxyprogesterone (17OHP) is a 21-carbon progestagen, produced by the adrenal gland (ACTH control) and gonads (LH or HCG control). 17OHP is a precursor to 11-deoxycortisol (11DOC) and is elevated in the most common form of congenital adrenal hyperplasia (CAH), 21-hydroxylase deficiency.
CAH is a group of inherited metabolic disorders of adrenal steroid hormone biosynthesis. The clinical features derive from a combination of under-production of either cortisol or aldosterone or both, and increased production of adrenal androgen precursors. The incidence of the classical disorder in Scotland is approximately 1/15,000.
Urine Cortisol
Cortisol is the major glucocorticoid hormone synthesised from cholesterol in the adrenal cortex. Synthesis is stimulated by the anterior pituitary adrenocorticotrophic hormone (ACTH), which is under control of the hypothalamic peptide, corticotrophin-releasing hormone (CRH).
As cortisol concentrations increase, the binding capacity of cortisol binding globulin in the circulation is exceeded, resulting in a disproportionate rise in urine cortisol concentrations. Urine cortisol measurement is useful as a screening test for cortisol excess (Cushing’s syndrome). Urine cortisol measurement can also be used as part of a dexamethasone suppression test. Multiple EMU cortisol measurements may also be useful in the investigation of possible cyclical Cushing’s.
Sample Requirements and Reference Ranges
- Sample Type: Urine (24 hr, random or early morning urine)
- Container: Plain urine container (no preservative)
- Precautions: None
- Minimum Volume: 10 mL
- Reference Range:
- Adults (EMU): <40 nmol/mmol creatinine
- Adults (24 hour): <165 nmol/24 hour
- Children (≤10 yrs): <40 nmol/mmol creatinine
- Turnaround Time: 14 days
- Method: Liquid chromatography-tandem mass spectrometry
- Quality Assurance: UK NEQAS
Urine Steroid Profile
A urine steroid profile includes all major metabolites of steroids, including glucocorticoids, mineralocorticoids and precursors.
The test is used to identify genetic disorders of steroid metabolism, though the screening or diagnostic test for congenital adrenal hyperplasia should be serum 17-hydroxyprogesterone. Steroid profiling is also useful to detect abnormal steroid secretion from adrenal and gonadal tumours.
Sample Requirements and Reference Ranges
- Sample Type: Urine (Aliquot of 24 hour urine for adults or children aged 11 and over; random for children <11 years)
- Container: Plain urine container (no preservative)
- Precautions: None
- Minimum Volume: 10 mL preferred. Smaller volume acceptable for babies (min. 2 mL).
- Reference Range: Age and sex dependent. Interpretation accompanies each report.
- Turnaround Time: 28 days
- Method: Gas chromatography-mass spectrometry
- Quality Assurance: Sample exchange programme
25-Hydroxy Vitamin D
Vitamin D is required for absorption of calcium and phosphate from the gut. The majority of vitamin D is produced in the skin when exposed to sunlight and the remainder obtained in the diet.
25-hydroxy vitamin D (25OHD) is the most abundant vitamin D metabolite in the circulation. It is relatively inactive but its measurement is the best indicator of vitamin D status. 25OHD exists in two forms, D3 and D2, and both are equally measured by the LC/TMS method.
Assessment of vitamin D status is important in patients with abnormal calcium or phosphate levels, possible osteomalacia and malabsorption, and osteoporotic patients before giving the first dose of IV bisphosphonates (to reduce the risk of drug induced hypocalcaemia).
NB. Request intervention procedures have been set up to reduce unnecessary testing. The request intervention interval for vitamin D is 340 days. All repeat requests within this period are reviewed by the Duty Biochemist and may be over-ridden if appropriate clinical details are provided.
Please refer to the NHSGGC Vitamin D Requesting and Prescribing Guidelines
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 500 μL (140 μL for neonates)
- Reference Range:
- <25 nmol/L: Vitamin D deficient, consider supplementation
- 25 – 50 nmol/L: Borderline low vitamin D, risk of secondary hyperparathyroidism, consider increase in vitamin D intake
- >50 nmol/L: Adequate vitamin D
- Turnaround Time: 14 days
- Method: Liquid chromatography-tandem mass spectrometry
- Quality Assurance: UKNEQAS
1,25-Dihydroxy Vitamin D
1,25-dihydroxy vitamin D (1,25DHD) is the active form of vitamin D, produced primarily by the kidney by hydroxylation of 25-hydroxy vitamin D. 1,25DHD is the form of vitamin D that stimulates resorption of calcium from bone, intestinal absorption and renal reabsorption.
NB. 1,25DHD should not be used to determine vitamin D status; 25-hydroxy vitamin D is the best marker for this purpose.
Indications for 1,25DHD are limited. Measurement may be useful in the investigation of possible vitamin D-dependent rickets and in patients with hypercalcaemia to investigate possible excess 1,25DHD production e.g. granulomatous diseases (sarcoidosis, TB or lymphoma).
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 250 μL
- Reference Range: 20 – 120 pmol/L (interim range pending further evaluation)
- Turnaround Time: 35 days
- Method: IDS iSYS
- Quality Assurance: DEQAS